product introduction
product description
Everyone suffers from back and knee pain at least once in their lifetime, one of the main reasons for which
is bad movement habits that cause damage to the spine and severe muscle spasms. We all suffer from daily
activities, sedentary life or fatigue in the back and knee area, which over time becomes a major challenge
in our daily lives. Therefore, the prevalence of these complications threatens the economy and health of the
world.
Now, if there is a device that, by nourishing the cells of damaged and weak tissues, causes nerve recovery,
reduces muscle tension and pain, the blood supply process to the vital organs of the body becomes easier,
which can help reduce pain and restore the health of people in society. Therefore, the goal of this project
is to help humanity.
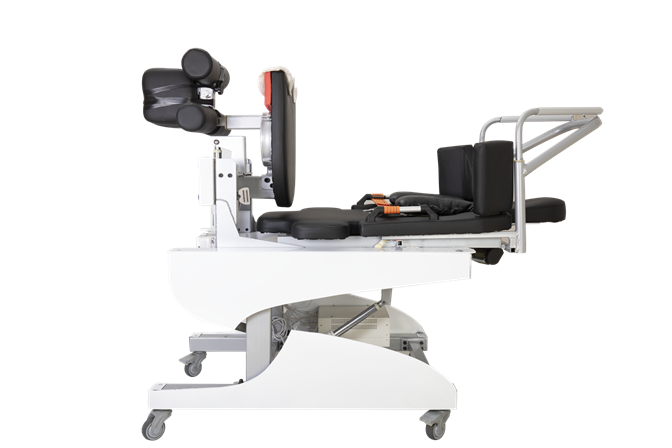
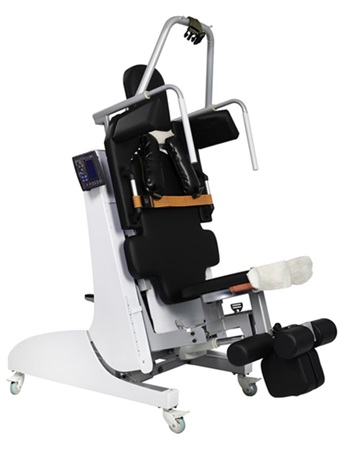
This device was designed and built for this purpose, to help with the application of superior technology to
nerve recovery and reduce nerve and muscle tension. This is done by creating hypoxia and ischemia of the
lower and mid-body tissues, which facilitates blood flow to the body's vital organs and has a direct impact
on reducing pain and ensuring people's health.
Being in a floating state reduces pressure on the spine, which enables cellular nutrition of damaged
tissues. This product is designed and manufactured electromechanically in line with the medical-sports and
rehabilitation industries, and its effectiveness has been evaluated by clinical trials on 800 people aged 15
to 85 who suffer from various types of back, neck, spine and knee pain, all of whom have reported
significant relief after use.
Its most important competitive advantage is the vertical traction axis with 11 specific missions, which
distinguishes this device from other relatively similar devices on the market.
In addition, it allows for simultaneous controlled traction of the neck, waist and knees in two separate
components.
On the other hand, due to fixing the lower body from the knee joint and the upper body from the armpit,
which creates a greater degree of freedom for stretching the desired area, this device is completely
non-invasive and safe, and ultimately minimizes the risk of injury for high-risk individuals.
Another advantage of it is that it is designed in two versions: home and clinical.
Also, the use of this device is not only for injured individuals. Rather, healthy individuals can also use
it to relieve fatigue and pain caused by daily activities for recovery and energy return. Therefore, it can
be used in all specialized centers such as hospitals, gyms and physiotherapy and public places such as
airports and hotels.
The unique design of this device has made it available to the public in the most optimal production mode.
In order to protect the environment, it can be said that
the production of this technology does not cause any pollution to the environment and is recyclable
helps reduce hospital waste resulting from surgical procedures
eliminates many drugs, chemicals, and painkillers
and contributes to the productivity and development of healthy and vibrant human resources.
The back and knee recovery device is the result of an innovative method of designing a specific exercise
based on the scientific and specialized genius of Professor Parvizi. This device is supported by the
professor's 35 years of experience as a player, coach, instructor, and professional bodybuilder for various
sports teams and is based on a special stretching method that has so far obtained the following valid and
scientific approvals from competent authorities:

1- Industrial design registration certificate from the Iranian Intellectual Property Center

2- Winning a silver medal in the World Inventors Federation member competitions

3- Publication (International Registration) in the United States Patent and Trademark Office under number PUB NU:2024/0342038AL / PUB DATE OC.17,2024
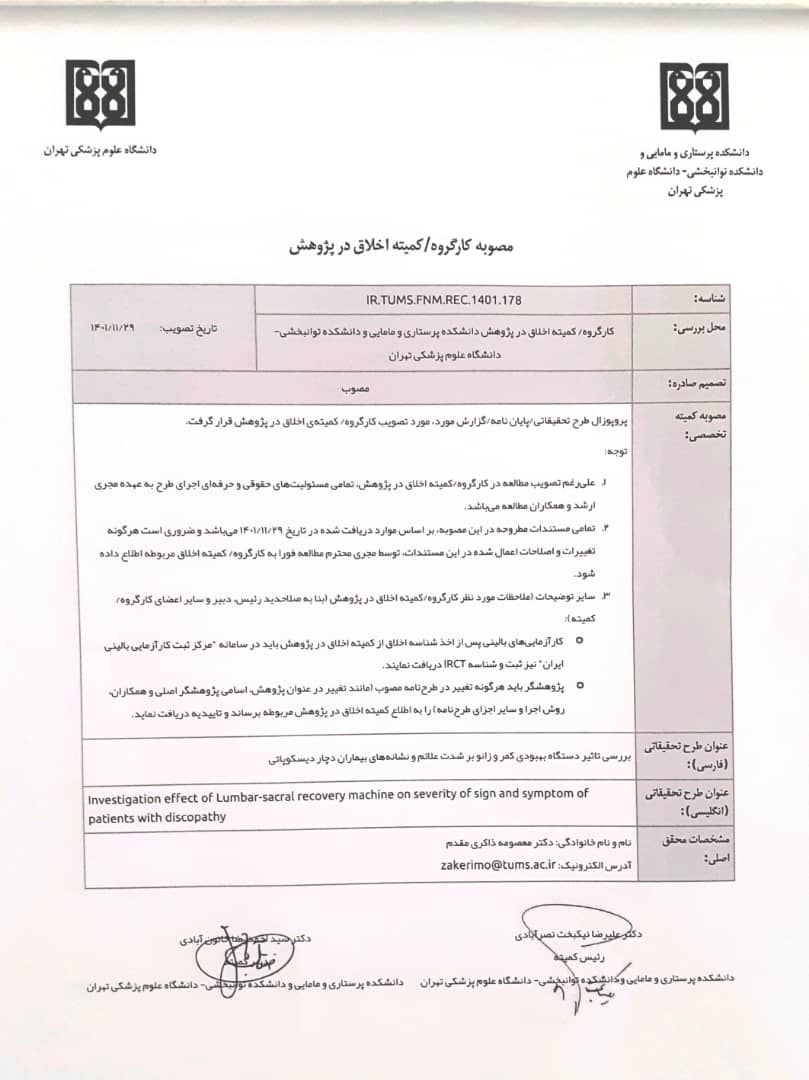
4- Obtaining the code of ethics under the number IR.TUMS.FNM.REC.014010178

5- Obtaining the clinical trial code number IRCT20191027045257N3

6- Registration of the application at the Swiss Patent Office under number PCT NU: PCT/IB2022/051262

7- Patent certificate from the Tehran Patent and Industrial Property Office

8- Technical and clinical approval from the Medical and Rehabilitation Equipment Technology Development Center, Tehran University of Welfare and Rehabilitation Sciences

9- Physical Education and Sports Sciences Research Institute, Ministry of Science, Research and Technology of Iran

10- A group of California chiropractors

11- Obtaining the top rank in the Medical Equipment Technology Exhibition of Islamic Azad Universities of Iran